Classifying the Decontamination Process
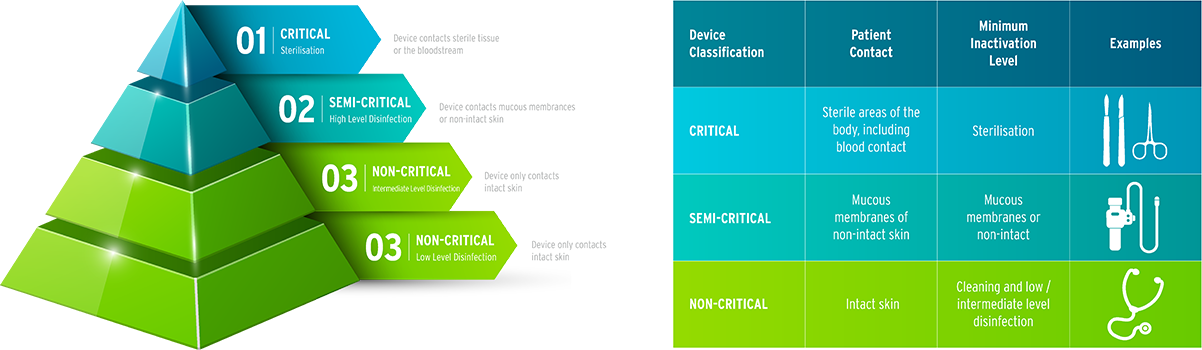
Classifying the decontamination process provides guidance in practicing safe infection control measures in healthcare. Further to this, the Spaulding classification of medical devices divides items into one of the following 3 categories to describe what level of disinfection is appropriate.
1. Critical Items
Critical items are those instruments that have been in contact with sterile tissue and vascular systems. This includes: surgical instruments, catheters & probes used in sterile body cavities. These items must be sterilised after use to eliminate the high risk they pose to transmission of infections. These medical devices can be steamed through use of steam sterilisation, treated with ethanol oxide, hydrogen peroxide gas plasma; or chemical sterilisation methods available.
2. Semi-Critical Items
Semi-critical medical devices are those that do not enter soft tissue or bone, however still make contact with mucous membranes or non-intact skin surfaces. This typically will include flexible endoscopes, respiratory & anesthesia equipment, esophageal manometry probes, anorectal manometry catheters and diaphragm fitting rings. These items will generally require sterilisation also. In some cases where sterilisation is not achievable, cleaning and high-level disinfection are carried out. Flexible endoscopes can undergo both high-level disinfection and sterilisation cycles with an Automated Endoscope Reprocessor (AER).
3. Non Critical Items
This includes any medical devices that are in contact with intact skin but not mucous membranes. These typically include stethoscopes, blood pressure cuffs and environmental surfaces such as patient furniture. These items do not pose a high-level risk of infection and typically only require low – intermediate levels of disinfection applied.